For situations wherever no established method is obtainable, careful scheduling and execution are needed to produce a strong method. Except for sample planning, there are actually 4 major actions to learn when developing an HPLC or UHPLC method:
, 2011); (ii) to ensure the particles are biocompatible and reach the tumor location devoid of staying identified via the immune procedure when Utilized in vivo
Behavioral marketing cookies allow for us to obtain details based on the observation of your respective searching patterns and behaviors on the internet, if you want in order to tell you about advertising and marketing articles that most closely fits your individual tastes and passions.
Dr. Helmut Schneider brings more than twenty years of knowledge towards analytical method development for biopharmaceuticals and compact molecules. For that previous twelve a long time, he combined this working experience Using the management of biopharmaceutical screening in equally R&D and GMP labs.
It truly is a good idea to check initial within the cellular phase. Many of the analytes ought to be absolutely soluble and the solution must be clear [32]. Diluent needs to be suitable Using the cell phase to get The great peak condition.
Preferably the flow price is fixed not over two.0 mL/moment. The stream which gives the least retention occasions, good peak symmetries, the very least again pressures, and much better separation of adjacent peaks/impurities could possibly be the picked as an optimized flow level to the Assessment.
Determined by the concerns above, the shipping coil composition was designed being a hollow multi-coil organized coaxially in shut proximity. This Improved the pliability of Procedure and application and expanded the opportunity purposes from the magnetic targeting therapy method to diverse objects being examined. Moreover, there was a substantial reduction in the peak of the person coils.
What on earth is Open up Entry? Open Obtain is an initiative that aims to help make scientific investigate freely available to all. To date our Group has remodeled 100 million downloads. It’s according to rules of collaboration, unobstructed discovery, and, most importantly, scientific progression.
, and a magnetic drug delivery program was carried out. The drug supply and MPI device created right here had been validated. Experimental benefits demonstrated that the SPIONs could proficiently be coupled to anti-tumor method development in pharma medication without compromising their potency, and that the created drug delivery method can successfully perform magnetic drug targeting enrichment and it is appropriate for observation of magnetic drug shipping by MPI.
In a single scenario, silicone was detected in a product following its container was altered. The original launch dimension exclusion method was inadequate as the silicone peak interfered Along with the detection of protein-connected impurities. To overcome the challenge, a method was made that sure the silicone towards the chromatography column even though the protein was allowed to pass through and become analyzed.
Analytical method transfer is often managed below a transfer protocol that information the parameters to generally be evaluated As well as the predetermined acceptance requirements that could be placed on the outcome.
As a result, these cookies do not need advertising needs, but only serve to generate our Site do the job better, adapting to our consumers normally. By activating them you will add to reported ongoing improvement.
Determined by the shipping examination experiments, it was evident the gradient magnetic field produced by the shipping coil structure must be equivalent more info to or larger than 270 mT, 3.2 T/m. The framework on the supply coil was developed according to this criterion.
Analytical method development is often a technique of proving that the developed chromatography method is suitable for its supposed use from the development and manufacturing of the pharmaceutical drug compound and drug solution.
 Haley Joel Osment Then & Now!
Haley Joel Osment Then & Now!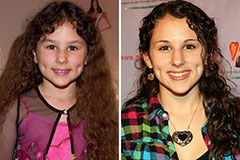 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Danica McKellar Then & Now!
Danica McKellar Then & Now!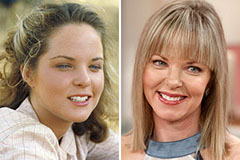 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Katey Sagal Then & Now!
Katey Sagal Then & Now!